Optimizing Excipient Use for Controlled Release: Strategies and Insights
Controlled release (CR) formulations have revolutionized the pharmaceutical industry by offering a host of benefits over immediate-release formats. These benefits include improved therapeutic outcomes, reduced dosing frequency, enhanced safety profiles, and minimized adverse effects. As a result, CR dosage forms are rapidly gaining prominence as one of the most dynamic delivery systems in the pharmaceutical landscape.
Key Excipients in CR Formulations
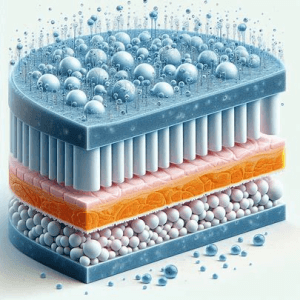
Hydroxypropyl methylcellulose (HPMC) is a crucial excipient in CR formulations. Its ability to form a swollen layer due to body temperature and its viscosity properties make it an ideal choice for matrix tablet formulations. Strategic HPMC substitution empowers formulators to create CR delivery systems with distinct performance characteristics.
Optimizing HPMC Properties
To optimize HPMC properties for enhanced CR performance, formulators must carefully consider the API solubility and HPMC matrix tablet performance. This involves understanding the nexus between HPMC viscosity and its ability to form a swollen layer. By navigating this nexus, formulators can create CR systems that achieve the desired release profiles.
Excipient Efficiency
The choice of excipients for CR formulations is critical. Excipients must be selected based on their ability to achieve the desired drug release profile. This often involves a trial-and-error approach, but recent studies have aimed to establish a rational basis for excipient selection. Factors such as pH, ionic characteristics, partition coefficients, and physical properties must be carefully considered to ensure the excipient matches the API and achieves the desired release profile.
Co-Processed Excipients
Co-processed excipients have shown great promise in CR formulations. These excipients are designed to provide a controlled-release system through direct compression. They offer advantages such as ease of implementation, solvent-free processing, and industrial scalability. The development of such excipients can help resolve the lack of new, functional excipients in the pharmaceutical industry.
Challenges and Complexities
Developing and manufacturing CR drug formulations come with several challenges. These include matching the suitable excipient with the drug, navigating intellectual property claims, and ensuring the drug formulation and excipients match the active to achieve the desired overall release profile. Additionally, different actives work well with different excipients, and the goal may be to release the drug immediately, over a specific time period.
Expert Insights
True L. Rogers, PhD, a senior lead scientist at IFF Pharma Solutions, emphasizes the importance of understanding the intricacies of CR formulations. He notes that CR performance is influenced by key excipient physiochemical properties, specifically HPMC viscosity and its ability to form a swollen layer due to body temperature. Rogers highlights the need for strategic HPMC substitution to create CR delivery systems with distinct performance characteristics.
Adriana Quiroga, application development and innovation leader at IFF Pharma Solutions, underscores the significance of HPMC in CR formulations. She emphasizes the importance of understanding the nexus between HPMC viscosity and its ability to form a swollen layer, as well as the need for careful selection of excipients based on their ability to achieve the desired drug release profile.
Conclusion
Optimizing excipient use for controlled release requires a deep understanding of the intricacies of CR formulations. This involves careful selection of excipients based on their ability to achieve the desired drug release profile, strategic HPMC substitution, and the development of co-processed excipients. By navigating the complexities of CR formulations, pharmaceutical companies can create innovative products that improve therapeutic outcomes, reduce dosing frequency, and enhance safety profiles.
“Controlled release performance is influenced by key excipient physiochemical properties, specifically HPMC viscosity and its ability to form a swollen layer due to body temperature.” — True L. Rogers, PhD
References
- Controlled Release Redefined: The Strategic Use of Excipients in Formulations
- Optimizing Excipient Properties to Prevent Aggregation in Biotherapeutic Formulations
- Excipient Efficiency for Controlled Release
- Navigating the Complexities of Controlled Release Drug Formulations
- Preparation of Co-Processed Excipients for Controlled-Release of Pharmaceuticals